Abstract
Introduction Thalassemia is an inherited anemia caused by diminished globin chain synthesis. More severe forms lead to ineffective erythropoiesis and chronic hemolytic anemia (HA) with subsequent clinical complications. Two types of thalassemia, alpha (α) and beta (β), result from defective synthesis of α- or β-globin, respectively. Limited research has been conducted in the US regarding burden of comorbidities, including both non-transfusion dependent thalassemia (NTDT) and transfusion dependent thalassemia (TDT), especially α-thalassemia.
Methods Claims data for patients and controls were selected from the MarketScan® Commercial and Medicare and Multi-State Medicaid claims databases. Patients were those with ≥1 inpatient or ≥2 claims in any setting for α- or β-thalassemia international classification of diseases (ICD)-9/ICD-10 codes from 1/1/2013 to 6/30/2021 (Table 1). Due to limitations in billing claims, some patients with thalassemia trait and minor may have been coded incorrectly by physicians and included in the NTDT group. The index date was defined as the first date of α- or β-thalassemia diagnosis code. Adults (≥18 years) with 12 months of continuous enrollment following index were included. TDT was defined as ≥8 transfusions within 12 months of follow-up, each within 42 days of each other. Controls with no history of thalassemia or other HAs were matched at 5:1 to thalassemia cases on age, sex, payer, and follow-up time. Medicaid patients were also matched on race. Thalassemia-related clinical outcomes, including pulmonary hypertension, cardiovascular disease, cerebrovascular disease, osteoporosis, and other comorbidities were measured during 12 months of follow-up and compared to matched controls. Chi-square (categorical variables) and t-tests (continuous variables) were used for outcome comparisons with a significance level of 0.05.
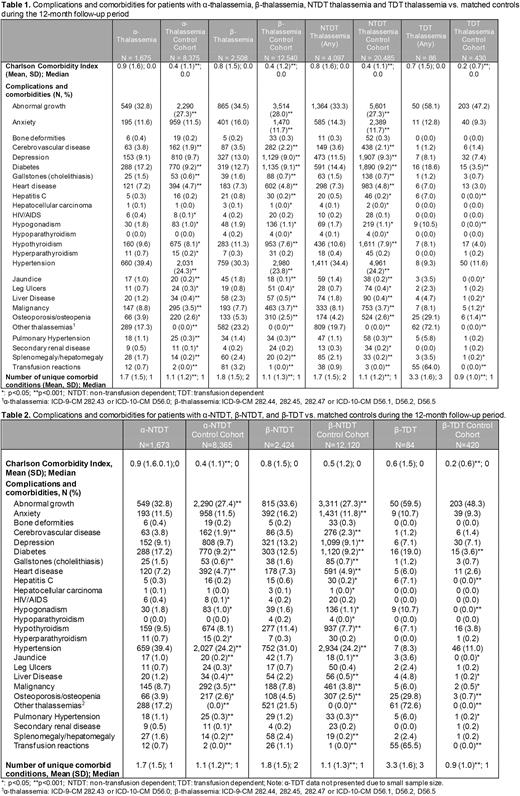
Results In the commercial and Medicare population, a total of 4,183 patients with thalassemia and 20,915 matched controls were analyzed. Of patients with thalassemia, 1,675 (40.0%) had α-thalassemia and 2,508 (60.0%) had β-thalassemia. In the 12 months following index, 2 (0.1%) α-thalassemia and 84 (3.3%) β-thalassemia had TDT; 1,673 (99.9%) α-thalassemia and 2,424 (96.7%) β-thalassemia had NTDT (Table 1). Sensitivity analysis without the 42-day requirement between transfusions had a minimal impact on TDT numbers. The average age for NTDT was 46.3 (SD 14.8) years and 29.4% were male. Compared to matched controls, α-NTDT and β-NTDT were more likely to have cardiovascular disease (α-NTDT: 7.2% vs. 4.7%; β-NTDT: 7.3% vs. 4.9%), cerebrovascular disease (α-NTDT: 3.8% vs. 1.9%; β-NTDT: 3.5% vs. 2.3%), pulmonary hypertension (α-NTDT: 1.1% vs. 0.3%; β-NTDT: 1.2% vs. 0.3%), jaundice (α-NTDT: 1.0% vs. 0.2%%; β-NTDT: 1.7% vs. 0.1%) and liver disease (α-NTDT: 1.2% vs 0.4%, β-NTDT: 2.2% vs. 0.5%) (all p<0.001) (Table 2). Osteoporosis (α-NTDT: 3.9% vs. 2.6% and β-NTDT: 4.5% vs. 2.5%; both p<0.005) and hypogonadism (α-NTDT: 1.8% vs. 1.0%, p=0.005; β-NTDT: 1.6% vs. 1.1%, p=0.045) were also more frequent among NTDT than matched controls. α-NTDT (1.7 [SD 1.5] vs. 1.1 [SD 1.2]) and β-NTDT (1.8 [SD 1.5] vs. 1.1 [SD 1.3]) had a higher mean number of comorbidities than their respective controls (both p<0.001; Table 1). β-TDT also had higher comorbidities compared to matched controls; there were too few patients with α-TDT to report. The Medicaid analysis of thalassemia compared to controls showed similar results to the Commercial/Medicare analysis, with a larger difference in the number of comorbidities observed (α-NTDT (2.3 [SD 1.8] vs. 1.1 [SD 1.4]) and β-NTDT (2.7[SD 1.8] vs. 1.3 [SD 1.5]; both p<0.001).
Conclusions Serious comorbidities and unmet needs persist for patients with thalassemia, even in thalassemia types that have historically been considered less severe such as NTDT. Both α-NTDT and β-NTDT had significantly higher clinical burden than matched controls including endocrinopathies, cardiovascular disease, liver disease and pulmonary hypertension - conditions associated with considerable morbidity and mortality. Some individuals with thalassemia minor or trait may have been included, suggesting that NTDT estimates in this study provide a lower boundary for the impact of NTDT. Additional therapies are needed to address the underlying cause of the disease, and for prevention of these serious complications.
Disclosures
Lombard:Agios Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Gilroy:Agios Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Li:Agios Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company, Other: Stockholder. Zhao:Agios Pharmaceuticals: Current Employment. Lew:Merative: Current Employment; IBM Watson Health: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Bullock:Merative: Current Employment; IBM Watson Health: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Davis:Merative: Current Employment; IBM Watson Health: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Sheth:Agios: Consultancy, Research Funding; Forma: Consultancy, Research Funding; Chiesi: Consultancy; Fulcrum: Consultancy; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bluebird Bio: Consultancy; CRISPR/Vertex: Other: Member of Clinical Trial Steering committee.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal